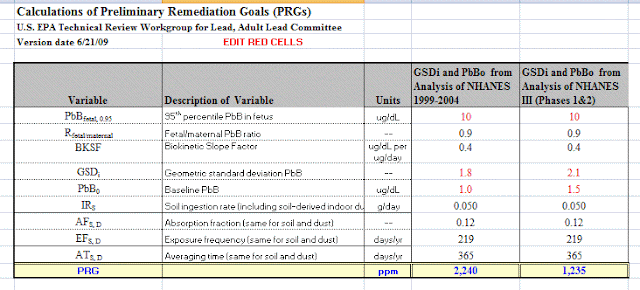
ATSDR recognizes that, at present, no single generally applicable procedure for exposure assessment exists, and, therefore, exposures to carcinogens are best assessed on a case-by-case basis with an emphasis on prevention of exposure. (1)What this means is that we can provide no real method for dose/response when it comes to exposure to chemicals suspected to be carcinogens. Therefore "0" exposure is recommended.
ATSDR recognizes that estimation of lifetime cancer risks is further complicated when available data are derived from less than lifetime exposures and that pharmacokinetic insights from animal models may be of utility in addressing this issue. (1)What this means is that we can look at animal models to help us, but we lack data on a lifetime exposure to chemicals (70 years) so our estimates of a cancer risk will be lacking.
The lowest dose levels associated with carcinogenic effects are identified as cancer effect levels (CELs), with the stipulation that such a designation should not be construed to imply the existence of a threshold for carcinogenesis. (1)Lowest dose found does not draw a line in the sand where below that is "no cancer" and above it is "cancer."
Also, exposures associated with upper- bound excess risk estimates over a lifetime of exposure (i.e., one case of cancer in 10,000 to one case of cancer in 10,000,000) as developed by EPA are presented. (1)We look at dose in terms of a cancer risk. CalEPA looks at a risk of cancer as one in 100,000 (10-5):
 |
| Source |
CalEPA has developed a "No Significant Risk Level" (NSRL) of 15 ug/day for lead. What this value is used for is to set a "safe harbor" amount for which the business does not have to post a Proposition 65 warning sign. It assumes that a person could consume up to 15 ug of lead per day (2.14E-03 mg/kg-day) with out a risk of more than 1 additional cancer per 100,000. 15ug of lead dos not assume a "safe" threshold, it is used to establish an amount of lead whereby a Proposition 65 notification is not required.
When Gradient states that lead exceeded the CalEPA NSRL by "11" times:
 |
| (Gradient 2011 Report) |
What exactly does a ratio of "11" mean in terms of risk? Here is what ATSDR says about assessing exposure to a carcinogen:
Both exposure and toxicity information are necessary to fully characterize the potential hazard of an agent. ATSDR considers exposure to an agent to be "an event consisting of contact at a boundary between a human and the environment at a specific environmental contaminant concentration for a specified interval of time; the units to express exposure are concentration multiplied by time." (1)Gradient assumes that each time a laundered shop towel is handled by a worker - exposure - there will be intake of the metals that remained on the towel after it has been washed. That intake is also referred to as a "dose" which ATSDR defines as:
"[t]he amount of contaminant that is absorbed or deposited in the body of an exposed individual over a specified time. Therefore, dose is different from, and occurs as a result of, an exposure." (1)In order for Gradient's model to be true, contaminants on the laundered shop towel must be deposited onto the hand and the hand must contact the mouth. The "dose" will be the amount of contaminant on the hand that is transferred into the mouth.
Cancer risk is looked at differently from chemicals that do not contribute to cancer or cause health effects other than cancer. Gere is what CalEPA says about exposure to carcinogens:
For chemicals that are listed as causing cancer, the "no significant risk level” is defined as the level of exposure that would result in not more than one excess case of cancer in 100,000 individuals exposed to the chemical over a 70-year lifetime. In other words, a person exposed to the chemical at the “no significant risk level” for 70 years would not have more than a “one in 100,000” chance of developing cancer as a result of that exposure. (2)How does CalEPA calculate that "no significant risk level” for lead?
 |
| Source Page 15 |
The "theoretical cancer potency" - qhuman - is also called the cancer slope factor.
 |
| Source |
NSRL = (0.00001 x 70) / 0.47 = 0.0148 mg or 0.0148 * 1000 = 14.8 ug ~ 15 ug (ppb).In other words, a person exposed to 15 ug (ppb) of lead for 70 years would not have more than a “one in 100,000” chance of developing cancer as a result of that exposure.
If we agree that 15 ug consumed for 17 years would show more than one additional cancer per 100,000, what would Gradient's intake of 2.4E-03 mg/kg-day (0.168 mg)?
 |
| (Gradient 2011 Report) |
In other words, if 15ug lead = 1 in 100,000, what would 0.168 mg lead result in?
Guess I'll need to calculate that. Dang, more math...and I suck at math. (Note to past self should time travel become a reality: Take math instead of computer programming in Fortran.)
Next post: Laundered Shop Towels: 13 - 100,000 workers using 12 towels per day
.