In the commercial/industrial setting, the most sensitive receptor is the fetus of a worker who develops a body burden as a result of non-residential exposure to lead. Based on the available scientific data, a fetus is more sensitive to the adverse effects of lead than an adult.
We assume that cleanup goals (preliminary remediation goals or PRGs) that are protective of a fetus will also afford protection for male or female adult workers.
The model equations were developed to calculate cleanup goals such that there would be no more than a five per cent probability that fetuses exposed to lead would exceed a blood lead (PbB) of 10 micrograms lead per deciliter of blood (µg/dL). This same approach also appears to be protective for lead’s effect on blood pressure in adult males.How much lead in soil consumed is necessary to produce 10 micrograms lead per deciliter of blood?
This is a fair and relevant question, and it relates to the problem with workers using laundered shop towels.
If Gradient is stating an "exceedance ratio" of "591" times the CalEPA MADL, which is the "maximum allowable dose levels for chemicals listed as causing birth defects or other reproductive harm" And the MADL for lead is based on "fetal development," then looking at how much lead consumed is required to produce "10 micrograms lead per deciliter of blood" - an actual quantitative amount of lead in the blood where we expect there will be adverse effects to the fetus above that level - would tell us how much total lead would need to be ingested to bring about that amount of lead in the blood.
Let's look at the variables EPA uses and the values they assigned:
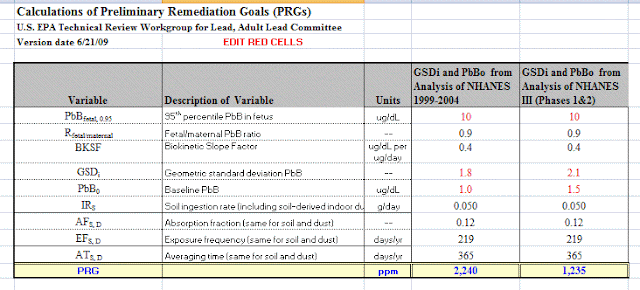 |
| EPA |
If 10,000 ppm is 1%, 2,240 ppm is 0.2%. So...0.2% of the soil consumed puts into the blood the amount of lead to achieve 10 micrograms lead per deciliter of blood.
How much total lead would be consumed if 50 milligrams of soil containing 2,240 ppm of lead is ingested?
0.2% * 50 mg = 0.002 * 50 = 0.1 mg of leadBased on a PRG of 2,240 ppm, EPA states:
A key concept is that a PRG is the average concentration of a chemical in an exposure area that will yield the specified target risk in an individual who is exposed at random within the exposure area. Thus, if an exposure area has an average concentration above the PRG, some level of remediation is needed. (1)The consumption of lead, and I am assuming from any source, of less than 0.1 mg per day, would not contribute to more than 10 ug/dl of lead in the blood, which is the amount of lead in the blood protective of a fetus.
What is the amount of lead per kg of body weight?
0.1 mg-day / 70 kg = 0.0014 = 1.4E-03 mg/kg - daySince lead is the one metal that produced the highest "Exceedance Ratios of Individual Toxicity Reference Values for Exposure via Hand Contact - Typical Use (12 Towels)," what is applicable to lead will also be applicable for all the other lower "exceedance ratios" Gradient reports. (2)
So..
The MADL value of 0.5 ug-day lead is for "safe harbor" reporting to the public. The EPA's PRG of 2,240 ppm (0.1 mg - day) is the clean up level for soil to yield a specified target risk of less than 10 ug/dl of lead in the blood deemed to be protective of the fetus. Both the MADL and PRG are based on protecting a fetus but are calculated based on different assumptions. The CalEPA's MADL assumes 1000 time less the NOAEL is "safe" for a fetus, and the EPA's PRG assumes a blood lead level of less than 10 ug/dL is "safe" for a fetus.
Which one appears to be a bit more sound?
How does the Gradient lead intake compare to the EPA PRG for lead?
0.0043 mg/kg-day (Gradient's intake value for lead)
0.0014 mg/kg-day (based on EPA's PRG of 2,240 ppm lead in soil)That's an "exceedance ratio" of 3 times the safe lead level consumed which would be protective of a fetus.
Remember, that's based on Gradient's mean lead concentration they reported (post), the transfer of 13% of the lead from the towel to the hand (post), the transfer of 13% of the lead on the hand to the mouth (post), 12 exposures per day, 245 days a year, performed for 40 years.
How does it look if an HTE of 6% is used? (post)
0.0019 mg/kg-day (Gradient's intake value for lead using 6% for the HTE)That's an "exceedance ratio" of 1.3 times the safe lead level consumed which would be protective of a fetus.
How does it look if the CalEPA hand to mouth calculation is used? (post)
0.0022 mg/kg-day (CalEPA calculation for lead)That's an "exceedance ratio" of 1.6 times the safe lead level consumed which would be protective of a fetus.
You may be tempted to tell me "See! It's still above a safe threshold using any of those calculations!" For which I will remind you that those intakes are based on a mean lead concentration on the towel of 100 mg/kg. Go to the report and look at the range of lead concentrations Gradient lists (Table 4 page 7).
Those values also assume that there will be a transfer of lead from a towel that was washed in water with soap and heat dried, equivalent to 13% of the lead evenly distributed on the towel from which 75% of the surface area comes in contact with the hand.
Oh, and it also assumes that this will happen 12 times a day, and that each time the hand will contact the mouth and some value of lead will be consumed.
All of those things must happen in order to get an "exceedance ratio" that is higher than the "safe" threshold.
There is one more thing that needs to be considered as well. The CalEPA equation calculates the intake per day. The PRG value of 2,240 ppm is based on exposure to the soil for a year.
In other words, we would expect (assume) a person coming in contact with soil containing 2,240 ppm of lead for one year, to produce a blood level of no more than 10 ug/dL. That ppm is based on an exposure frequency (EF) of 219 days and an averaging time (AT) of 365 days.
This EPA PRG model assumes that there will not be constant exposure to soil containing lead, but assume there will be 219 days of exposure and 0.05 grams of soil will be consumed each time there is exposure. Based on this, 2,240 ppm of lead or less is deemed to be protective.
For an exposure period of one day, lead intake during one work day (12 towels in 8 hours) - using CalEPA's equation (see post) - can be calculated as follows:
- Intake = 0.0013 mg/cm2 x 19 cm2 x 0.5 x 1.5/hour x 8 hours = 0.148 mg per work day
- 0.148 mg per day = 0.148 / 70 kg = 0.0022 mg/kg-day Intake or 2.2E-03 mg/kg-day
- (0.0022 mg/kg-day * 245 days) / 365 days = 0.0015 mg/kg-day or 1.5E-03 mg.kg-day
If this same worker was exposed to soil contaminated with lead, the intake of lead would need to be less than 0.0014 mg/kg-day.
Using CalEPAs calculation, Gradient's values, and a 365 day averaging time, the "exceedance ratio" will be 0.0015/0.0014 = 1.07, or 1.1, or 1 = even. This average amount of lead reported by gradient will produce a blood lead level of less than 10 ug/dL even when 12 towels are used and the worker places the finger and palm to the mouth each time a towel is used. This is protective of the worker because it is protective of the fetus!
So the question now is: If lead had the highest "exceedance ratio" reported by Gradient, and you are now presented with CalEPA apples and EPA apples to compare Gradient apples with, are you still concerned about laundered shop towels creating any risk of a reproductive health concern for a worker, even a pregnant one?
Well...
Cancer!!! What about the risk of cancer?
Next post: Laundered Shop Towels: 12 - Everything gives you cancer...except laundered shop towels.
.
No comments:
Post a Comment